Our research has been focused on the synaptic physiology and synaptic plasticity of auditory brain circuits and neurons. We have discovered novel synaptic mechanisms in the auditory brainstem, thalamus and cortex, as well as their effects in normal and pathological processing in animal models of hearing loss, tinnitus and hyperacusis. Importantly, we are pursuing drug discovery/development and gene therapy for tinnitus, hyperacusis, and congenital hearing loss.
 The Santa Maria Lab is focused on tackling chronic suppurative otitis media (CSOM). CSOM is a chronically discharging hole in the eardrum, which produces hearing loss in more than 50% of cases and is associated with poor language and development. According to the World Health Organization, up to 330 million individuals suffer from CSOM-associated hearing loss worldwide. It accounts for 28,000 deaths yearly, a disease burden of over 2 million disability adjusted life years, and it is the most common cause of persistent hearing impairment among children in developing countries.
The Santa Maria Lab is focused on tackling chronic suppurative otitis media (CSOM). CSOM is a chronically discharging hole in the eardrum, which produces hearing loss in more than 50% of cases and is associated with poor language and development. According to the World Health Organization, up to 330 million individuals suffer from CSOM-associated hearing loss worldwide. It accounts for 28,000 deaths yearly, a disease burden of over 2 million disability adjusted life years, and it is the most common cause of persistent hearing impairment among children in developing countries.
The problem occurs in up to 40% of indigenous populations. If we can solve this problem, we can restore hearing to hundreds of millions around the world. We have already developed a therapy to regenerate the tympanic membrane, which will be in clinical trials soon. We have identified several novel mechanisms in pathogenesis and are identifying how people lose sensory hearing with infection. We are also developing our own novel therapeutics to clear ear infections.
Other diseases we are fighting include oral wound healing problems that occur after tonsillectomy. Tonsillectomy remains one of the most common surgeries performed around the world. We discovered a novel mechanism that leads to reducing pain and bleeding after surgery, and we are translating a new therapy to prevent complications. Oral mucositis occurs in over eighty percent of patients having chemoradiotherapy for head and neck malignancy, often leading to aborting curative therapy that could treat a patient’s cancer. We are translating a novel therapeutic that can prevent this from occurring so that patients can have reduced pain and suffering during cancer treatment.
Having made novel discoveries of how wound healing occurs in the ear, we’re investigating how we can repurpose our discoveries to other tricky problem areas, including preventing bleeding after a tonsillectomy, oral mucositis after chemoradiotherapy for head and neck malignancy, and oral aphthous ulcers.
 Dr. Thanos Tzounopoulos is a leading researcher in the field of auditory neuroscience and hearing research. His work at the Pittsburgh Hearing Research Center focuses on understanding the brain mechanisms behind normal and pathological hearing, such as in hearing loss, tinnitus, and hyperacusis.
Dr. Thanos Tzounopoulos is a leading researcher in the field of auditory neuroscience and hearing research. His work at the Pittsburgh Hearing Research Center focuses on understanding the brain mechanisms behind normal and pathological hearing, such as in hearing loss, tinnitus, and hyperacusis.

Dr. Gregory Basura is prominent researcher in the field of tinnitus at the University of Pittsburgh. The Basura Lab focuses on understanding how different sensory systems in the brain interact and contribute to tinnitus.
1. Multisensory Integration: Dr. Basura studies how the brain's auditory system interacts with other sensory systems, such as tactile inputs and body position. He uses animal models to investigate how these interactions can affect tinnitus.
2. Brain Plasticity: His research also explores brain plasticity, which is the brain's ability to adapt and change. He examines how the brain's auditory pathways change in response to tinnitus and single-sided deafness. This helps to understand how tinnitus develops and persists.
3. Functional Near-Infrared Spectroscopy (fNIRS): Dr. Basura uses a non-invasive imaging technique called fNIRS to study brain activity in humans with tinnitus. This technology helps him measure brain activity in people with tinnitus and single-sided deafness and how it changes when exposed to different stimuli.
4. Clinical Applications: His research aims to translate these findings into clinical applications such as drugs or devices. By understanding the brain mechanisms behind tinnitus, he hopes to develop better diagnostic tools and treatments for people suffering from this condition.
Dr. Basura's research is focused on understanding the complex interactions between different sensory systems in the brain and how they contribute to tinnitus. This knowledge is crucial for developing effective treatments and improving the quality of life for those affected by tinnitus.

Dr. Bharadwaj directs the Systems Neuroscience of Auditory Perception Lab (SNAPlab), a multidisciplinary team studying how our ears and brains encode and analyze complex sounds, such as speech, in real-world environments with multiple competing sound sources. A key focus is understanding the physiological determinants of ‘suprathreshold’ hearing problems—cases where individuals can detect sounds but struggle to make sense of them, particularly in noisy settings like crowded restaurants, busy streets, and other social environments. While hearing loss is known to reduce the audibility of soft sounds, far less is understood about the biological mechanisms underlying suprathreshold hearing challenges. 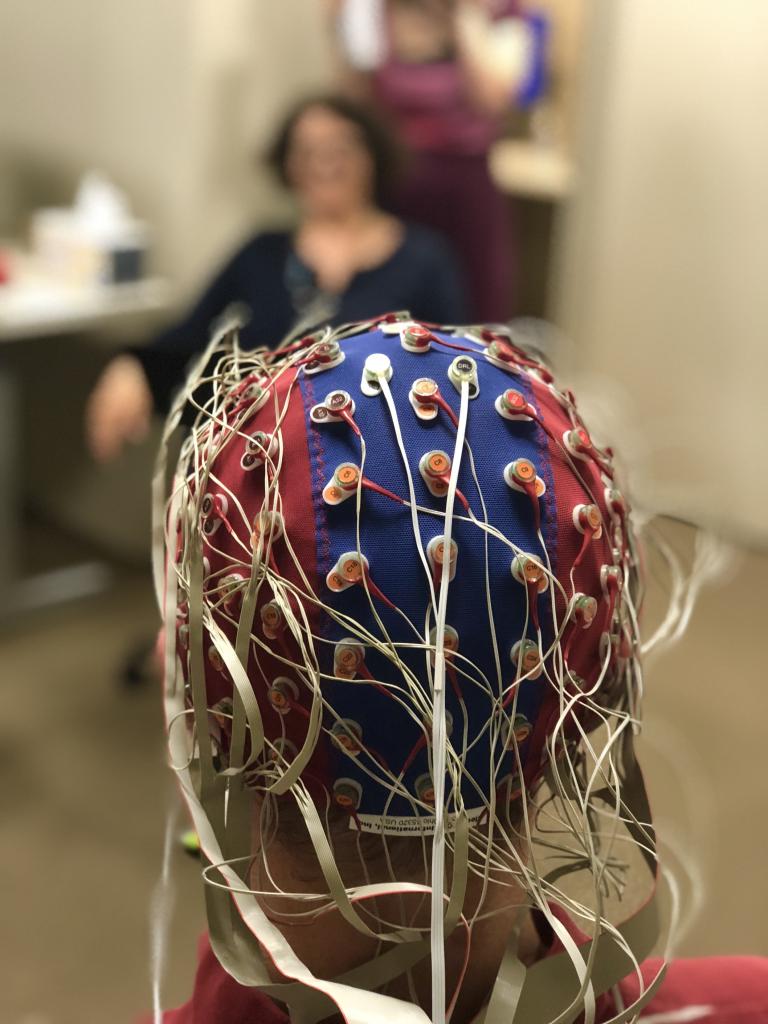 These challenges are widespread, affecting communication and quality of life for an estimated 1.5 billion people worldwide and costing the global economy nearly a trillion dollars. Even with state-of-the-art, clinically prescribed hearing aids that restore audibility, many still struggle with reduced speech clarity and difficulty filtering out competing sounds. SNAPlab leverages psychoacoustics, non-invasive physiological measurements (e.g., EEG, otoacoustic emissions), and computational modeling to gain insights into the biological mechanisms of successful hearing in noise and how they break down in hearing loss and neuropsychiatric conditions. The lab also collaborates extensively with researchers using animal models to probe neural mechanisms and with clinicians to translate findings into clinical applications. Through this integrative approach, SNAPlab aims to advance precision diagnostics for hearing problems, guide personalized interventions, and improve hearing aids, cochlear implants, and brain-machine interfaces.
These challenges are widespread, affecting communication and quality of life for an estimated 1.5 billion people worldwide and costing the global economy nearly a trillion dollars. Even with state-of-the-art, clinically prescribed hearing aids that restore audibility, many still struggle with reduced speech clarity and difficulty filtering out competing sounds. SNAPlab leverages psychoacoustics, non-invasive physiological measurements (e.g., EEG, otoacoustic emissions), and computational modeling to gain insights into the biological mechanisms of successful hearing in noise and how they break down in hearing loss and neuropsychiatric conditions. The lab also collaborates extensively with researchers using animal models to probe neural mechanisms and with clinicians to translate findings into clinical applications. Through this integrative approach, SNAPlab aims to advance precision diagnostics for hearing problems, guide personalized interventions, and improve hearing aids, cochlear implants, and brain-machine interfaces.

The Cunningham Lab is interested in understanding the sensory and neural biology of the vertebrate auditory system, and in developing biological therapies for hearing loss. Many unique and highly specialized proteins with exquisitely precise subcellular localizations are critical for each step of sound processing. Hearing loss is the most common sensory deficit, and multiple forms of hearing loss involve aberrant assembly, trafficking, and/or regulation of key auditory proteins. We utilize mouse models of human deafness for our experiments. The similarities between the rodent and human auditory systems allow for a panoply of experimental manipulations that aim to uncover basic biological mechanisms and translational insights relevant for human health. 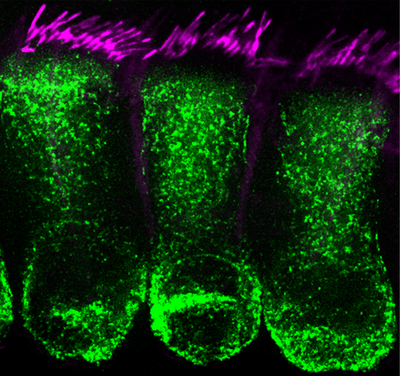 The lab utilizes cutting-edge techniques including the generation and analysis of novel genetic mouse models combined with biochemistry, molecular biology, histology, viral vectors and high-resolution fluorescent microscopic imaging. Ultimately, we hope to utilize our findings toward the development of new therapies for hearing loss and deafness. To this end, we are very interested in developing gene therapy strategies that can treat hearing loss. Dr. Cunningham, along with Drs. Tzounopoulos and Zevallos from the Department of Otolaryngology-HNS, have recently co-founded a startup company called Echogenesis Therapeutics, focused on developing AAV-based gene therapies for individuals with genetic forms of hearing loss.
The lab utilizes cutting-edge techniques including the generation and analysis of novel genetic mouse models combined with biochemistry, molecular biology, histology, viral vectors and high-resolution fluorescent microscopic imaging. Ultimately, we hope to utilize our findings toward the development of new therapies for hearing loss and deafness. To this end, we are very interested in developing gene therapy strategies that can treat hearing loss. Dr. Cunningham, along with Drs. Tzounopoulos and Zevallos from the Department of Otolaryngology-HNS, have recently co-founded a startup company called Echogenesis Therapeutics, focused on developing AAV-based gene therapies for individuals with genetic forms of hearing loss.

In the Insanally Lab, our goal is to understand the neural basis of auditory learning, perception, and plasticity. As we’ve all experienced, sounds encountered in daily life are rarely neutral: you may not notice the sound of a honking horn while stuck in traffic but one heard while crossing the street might startle you. How do we learn to interpret what we hear and act on it? Atypical learning and the inability to flexibly navigate sensory environments are principal hallmarks of several neurological disorders including hearing loss, autism, attention disorders, and schizophrenia. Thus, understanding the neural basis of auditory perception and learning is essential for developing targeted therapies for improving patient outcomes. To address this critical need, my lab leverages cutting-edge approaches to investigate how the brain constructs flexible neuronal representations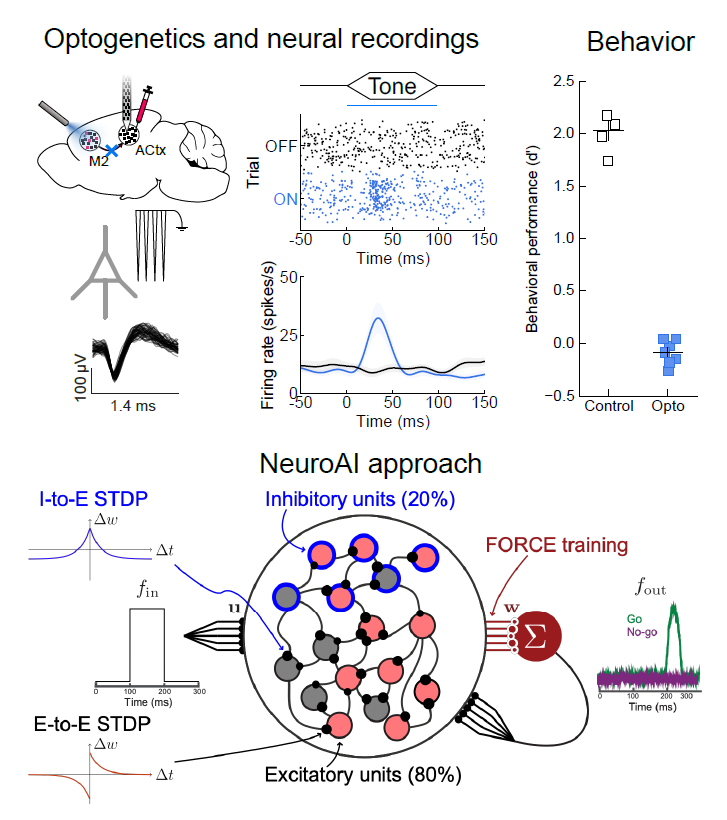 during auditory perception and learning and how the neural dynamics underlying these behaviors is disrupted in hearing disorders. Combining advanced genetic tools for probing and dissecting neural circuits, with state-of-the-art neural recording techniques that allow us to monitor the activity of thousands of neurons,and Neuro-AI based computational approaches, we seek to unravel how auditory perception and learning are implemented by brain-wide circuits.
during auditory perception and learning and how the neural dynamics underlying these behaviors is disrupted in hearing disorders. Combining advanced genetic tools for probing and dissecting neural circuits, with state-of-the-art neural recording techniques that allow us to monitor the activity of thousands of neurons,and Neuro-AI based computational approaches, we seek to unravel how auditory perception and learning are implemented by brain-wide circuits.

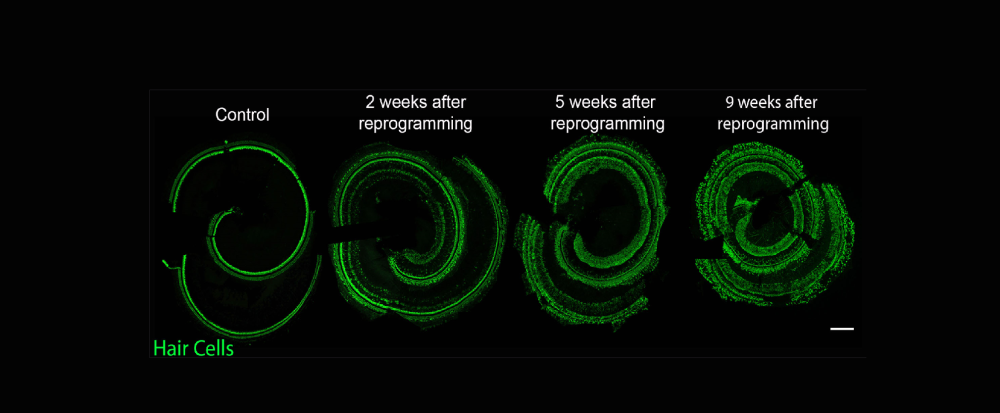
The McGovern Lab investigates the potential of the mammalian inner ear to regenerate following the loss of sensory cells that detect and transduce sound from the environment to the brain. These highly specialized cells are critical for our perception of the auditory world, but, unlike birds and fish, the mammalian inner ear has a very limited capacity to regenerate these cells and only early in development. The mature organ does not have any known capacity to naturally regenerate lost cells, and therefore our lab deploys transcription factors in order to reprogram lost sensory cells from their neighbors that remain in the ear.
We use genetically modified mouse lines that modify the transcriptome of cochlear cells specifically in mature non-sensory cells of the ear and investigate these through high resolution fluorescence histology and molecular genetic mechanisms. We are interested in understanding the circumstances necessary to reprogram non-hair cells into functional hair cells so that we can begin to design gene therapies for hearing restoration.

T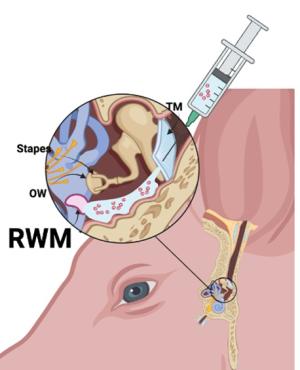 he primary challenge for systemic delivery to the inner ear is achieving a therapeutic dose across the blood-labyrinth barrier without causing systemic side effects. Local administration routes, such as intratympanic and intracochlear methods, present alternatives that bypass blood-labyrinth barrier issues encountered with systemic administration. However, these local methods come with drawbacks, including temporary or permanent hearing loss or low efficiency.
he primary challenge for systemic delivery to the inner ear is achieving a therapeutic dose across the blood-labyrinth barrier without causing systemic side effects. Local administration routes, such as intratympanic and intracochlear methods, present alternatives that bypass blood-labyrinth barrier issues encountered with systemic administration. However, these local methods come with drawbacks, including temporary or permanent hearing loss or low efficiency.
Our lab addresses the significant challenges inherent in developing effective inner ear substance delivery systems. One key hurdle involves not knowing the exact drug formulation for which the delivery system is being designed, affecting drug bioavailability and biodistribution. Another obstacle lies in translating promising findings from preclinical studies, conducted on animal models, into applicable solutions for human patients. Some of the current research projects to address these challenges are listed below:
- Developing ex-vivo and in-vivo models of drug delivery in large animals such as pigs similar to humans.
- Developing extracellular vesicles as efficacious and safe carriers to deliver therapy-related substances to the inner ear.
- Identifying novel sensory receptors and channels of round window membrane that sense chemicals, investigating how receptors and channels regulate drug passage to the inner ear, and harnessing receptors and channels to improve inner ear drug delivery.
- In vivo and ex-vivo 3D high-resolution imaging for inner ear visualization, metabolomics, inflammation, and drug biodistribution.
- Evaluate promising regeneration therapy effects delivered safely in a clinically relevant animal model like pigs.
- Nanomaterials and smart materials to improve drug delivery to the inner ear.

The primary interest of the Parthasarathy Lab for Translational Auditory Neuroscience is in understanding how the peripheral auditory system and the central auditory pathway interact in various forms of hearing loss. The research program integrates study of human clinical populations and animal models, using non-invasive, EEG-like evoked potentials as the translational bridge. The overall goal is to inform diagnosis and track the benefits of interventional therapies in clinical populations with hearing loss by utilizing insights obtained from animal models with similar forms of pathology.
 The Williamson Laboratory of Neural Circuits & Behavior in Health and Disease is dedicated to understanding how the flow of sensory information through brain-wide neural networks gives rise to purposeful behavior in models of health and disease. Using a variety of cutting-edge experimental and theoretical systems neuroscience tools to monitor, manipulate, and model the neural circuits of awake, behaving mice, we investigate brain-wide circuits in several different contexts:
The Williamson Laboratory of Neural Circuits & Behavior in Health and Disease is dedicated to understanding how the flow of sensory information through brain-wide neural networks gives rise to purposeful behavior in models of health and disease. Using a variety of cutting-edge experimental and theoretical systems neuroscience tools to monitor, manipulate, and model the neural circuits of awake, behaving mice, we investigate brain-wide circuits in several different contexts:
Perceptual decision-making, learning, and state-dependent dynamics. Mice learn to make decisions based on the identity of auditory stimuli. We seek to identify which neural circuits are involved, what auditory information is propagated brain-wide, what neural computations take place across learning, and how this information is modulated through top-down interactions from higher-order areas. We also seek to identify how a fluctuating internal state impacts the ability to make sensory-guided decisions.
Sensory-guided foraging. Mice are tasked with using auditory cues to forage for hidden targets (resources or prey). These freely-moving, naturalistic behaviors are combined with chronically implanted technologies that facilitate the recording of massive neural populations so that we can identify the neural computations that take place as mice navigate complex environments.
Acoustic communication. Multiple mice are allowed to interact with one another in a naturalistic setting. By combining neurophysiological approaches with video/sound tracking, we can accurately record and assign sound to space (to accurately track ongoing bouts of vocalizations) and identify which neural circuits contribute to social behaviors that rely on acoustic communication (courtship, for example), as well as probing how communication deficits manifest in disease.
Brain-wide propagation of auditory hallucinations. Auditory hallucinations feature prominently in many psychiatric disorders. We utilize targeted noise exposures to induce auditory hallucinations in mice before engaging in auditory-guided behaviors. By longitudinally monitoring distinct neural populations, we aim to track cognitive deficits across learning, correlate these cognitive deficits with neural activity, and use cell-type specific manipulation strategies to alter the brain-wide propagation of hallucinatory signals to reverse these cognitive deficits.

Dr. Xia has dedicated her career to hearing research, focusing on cochlear physiology and mechanics in various animal models, including mice, gerbils, and chickens. She has developed a transgenic mouse model, investigated gene regulation in the cochlea, and contributed to advancements in gene therapy for inner ear disorders.
 Since 2017, Dr. Xia has collaborated with Dr. Peter Santa Maria as a co-investigator on an R01 grant, as well as on several SPARK programs, leading a group of researchers studying cochlear immunity mechanisms. Together, they investigate cochlear immune responses to sensorineural hearing loss caused by chronic suppurative otitis media, noise-induced hearing loss, and autoimmune or immune-mediated inner ear diseases. Dr. Xia is also an expert in topical drug delivery, drug kinetics, and drug ototoxicity screening.
Since 2017, Dr. Xia has collaborated with Dr. Peter Santa Maria as a co-investigator on an R01 grant, as well as on several SPARK programs, leading a group of researchers studying cochlear immunity mechanisms. Together, they investigate cochlear immune responses to sensorineural hearing loss caused by chronic suppurative otitis media, noise-induced hearing loss, and autoimmune or immune-mediated inner ear diseases. Dr. Xia is also an expert in topical drug delivery, drug kinetics, and drug ototoxicity screening.

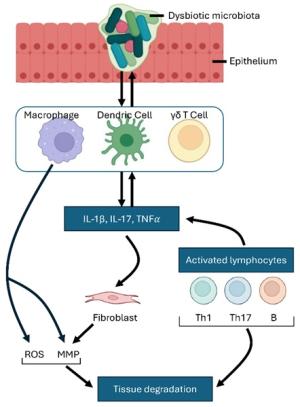
Vincent Yuan's translational research focuses on the mechanisms and treatment of chronic suppurative otitis media (CSOM) and Meniere's disease (MD), particularly from an immunological perspective, investigating both innate and adaptive immune responses. His studies examine how immune cells, such as macrophages and dendritic cells, are activated to produce proinflammatory mediators like TNFα and IL-1β. These cells not only drive inflammation but also influence the differentiation of T helper (Th) cell subsets, further escalating the immune response.
Additionally, Yuan's research explores the destructive effects, cell-cell crosstalk, and key signaling pathways activated by macrophages. A significant focus of his work is the role of the NLRP (NOD-like receptor protein) family in these processes. He investigates how NLRP proteins regulate inflammatory pathways that exacerbate chronic ear infections and inner ear disorders like MD. By exploring the interactions between innate and adaptive immune cells, Yuan aims to uncover critical mechanisms underlying unresolved inflammation in CSOM and MD, with the goal of identifying novel therapeutic targets.
